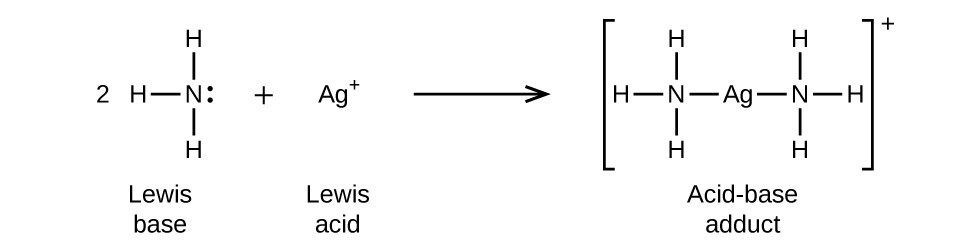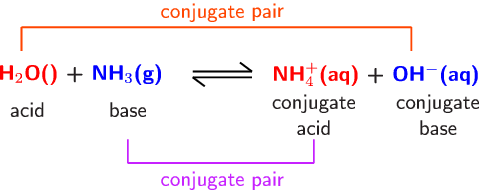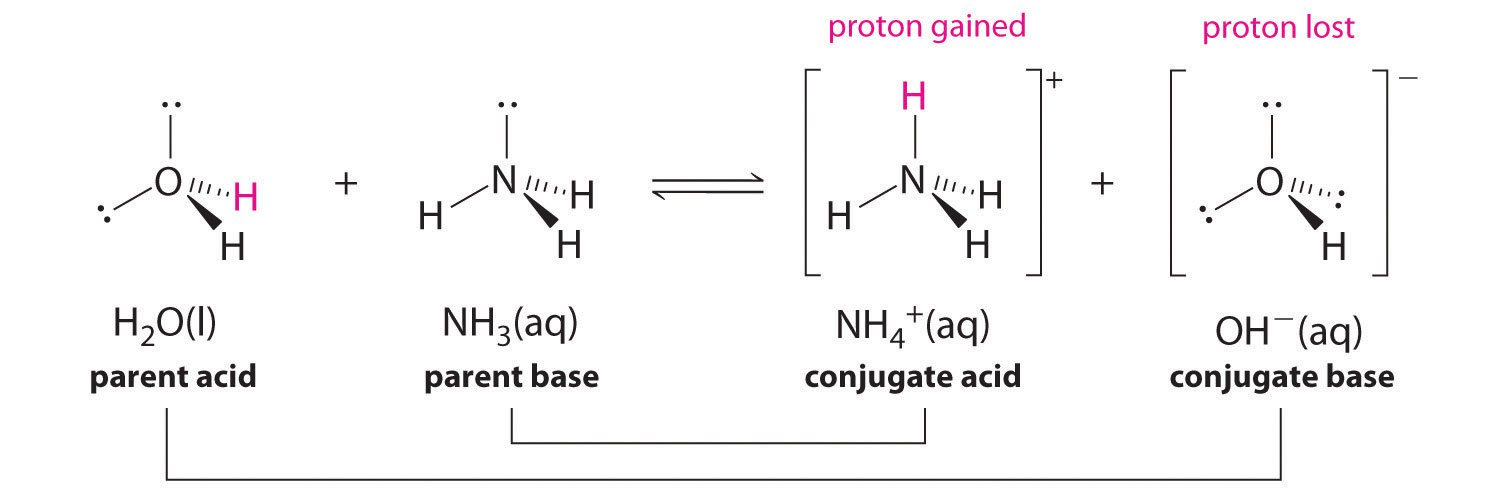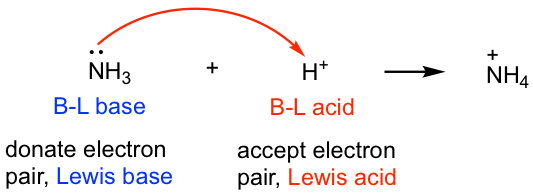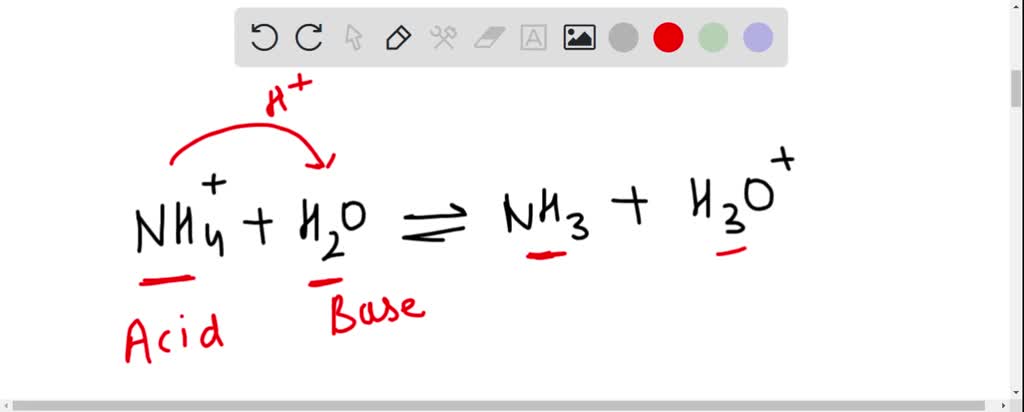
SOLVED: In the following reaction: NH4+ + H2O = NH3 + H3O+ A) H2O is a base and NH3 is its conjugate acid B) NH4+ is an acid and H20 is its

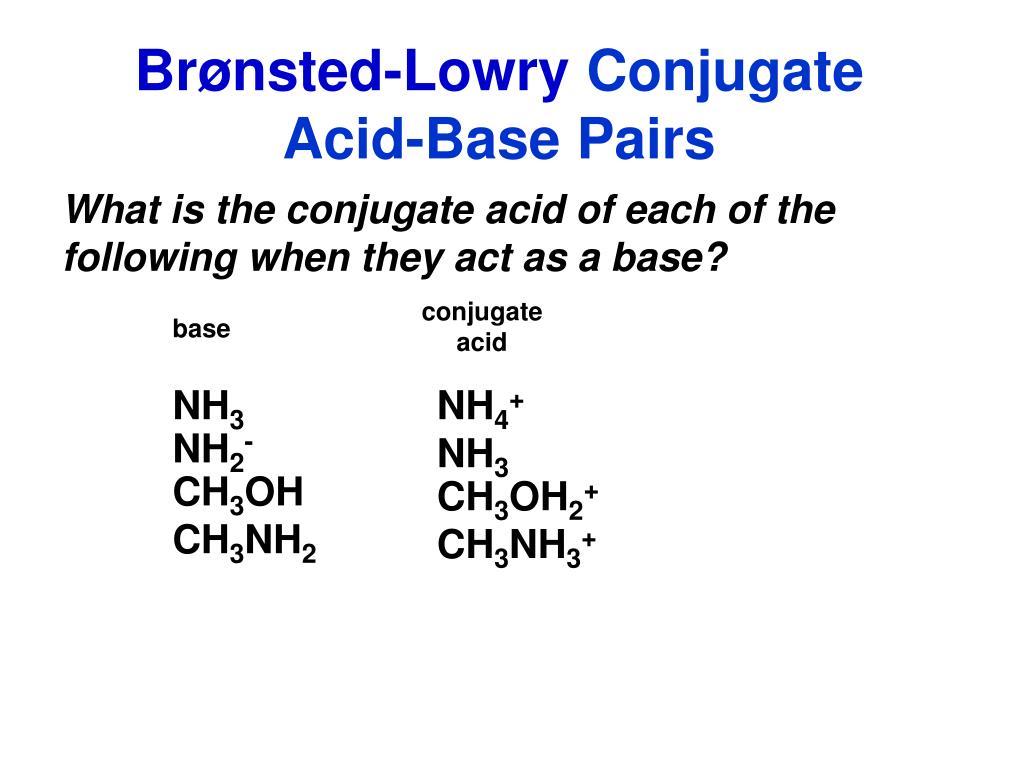
Identify the conjugate acid-base pairs in this equilibrium. NH3(aq) + H2S(aq) arrow HS-(aq) + NH4+(aq) | Homework.Study.com

NH3 is a weak base (Kb = 1.8 times 10^-5) and so the salt NH4Cl acts as a weak acid. What is the pH of a solution that is 0.050 M in